Optical force-based cell sorting and orientation
Motivation
In cell biology and biotechnology, optical tweezers are the first choice for optical manipulation such as sorting or orientation of biological objects, since they offer contactless, non-damaging, and flexible forces on a specimen. Especially for studies on signaling pathways within or between cells, a natural, three-dimensional confirmation is essential. Therefore, a high-resolution microscope is equipped with a powerful and flexible optical tweezers setup.
Sorting of suspension cells
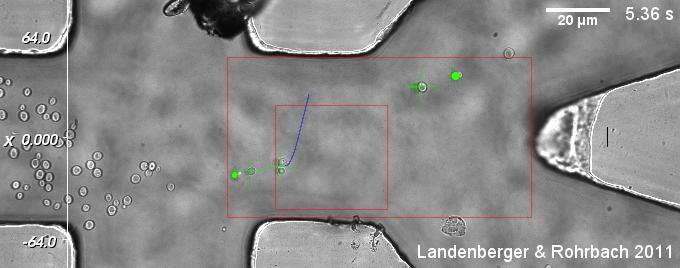
With an additional microfluidic chamber, very different kinds of suspension cells can be sorted by multiply criteria, which can be extracted from CCD camera images. These criteria, such as intensity of fluorescence signal and morphological properties like size or shape, can be evaluated and used for classification during a single run.
| Fig. 1: A suspension of yeast cells is sorted with dynamic optical tweezers. Each cell is classified by (i) a certain threshold value in the fluorescence channel and (ii) an appropriate size in the bright field channel. Sorted cells leave towards to upper right outlet, while unsorted cells continue their way to the lower outlet. (Click on the image to see the movie!) |
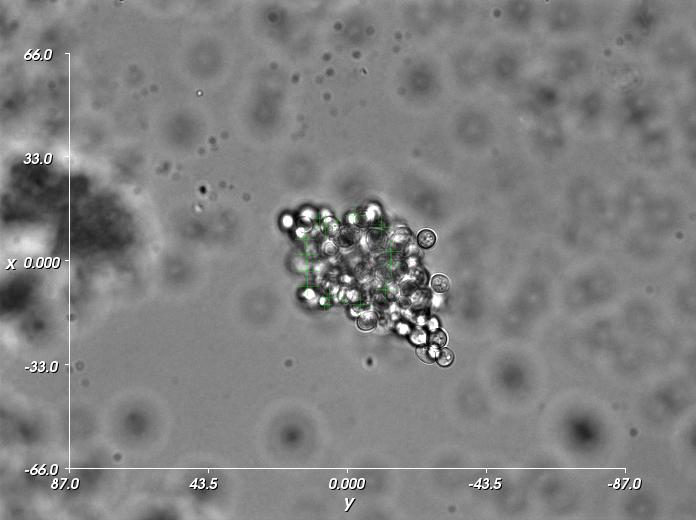
Orientation of large cell clusters
For orientation of large cell clusters, strategies must be developed which result in a maximum of optical force (with limited laser power) on a complex, irregular object. Especially for movements perpendicular to the observation plane, suitable locations on the cluster must be identified and the efficiency of each optical trap at each location must be controlled online. A spatial light modulator (SLM) and a fast hologram calculation algorithm are used to position many optical traps in 3D.
Support
This project is supported by the Excellence Initiative of the German Federal and State Governments (EXC 294). 
Collaboration
Carl Zeiss MicroImaging GmbH, Jena
Laboratory for MEMS Applications (IMTEK)
Related Publications
B. Landenberger, H. Höfemann, S. Wadle, and A. Rohrbach, Lab on a chip, 2012, 3177–3183.